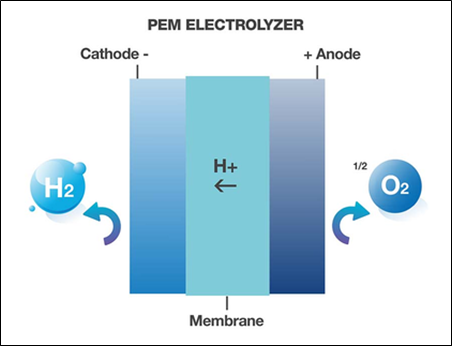
PEM
Pure water is fed into the anode of the electrolyzer, where it is split by a catalyst. Oxygen is produced directly on the anode side, whereas the hydrogen ions are passed through a solid polymer membrane and react on the cathode side to form hydrogen.